Dedicated To Current Good Manufacturing Practice
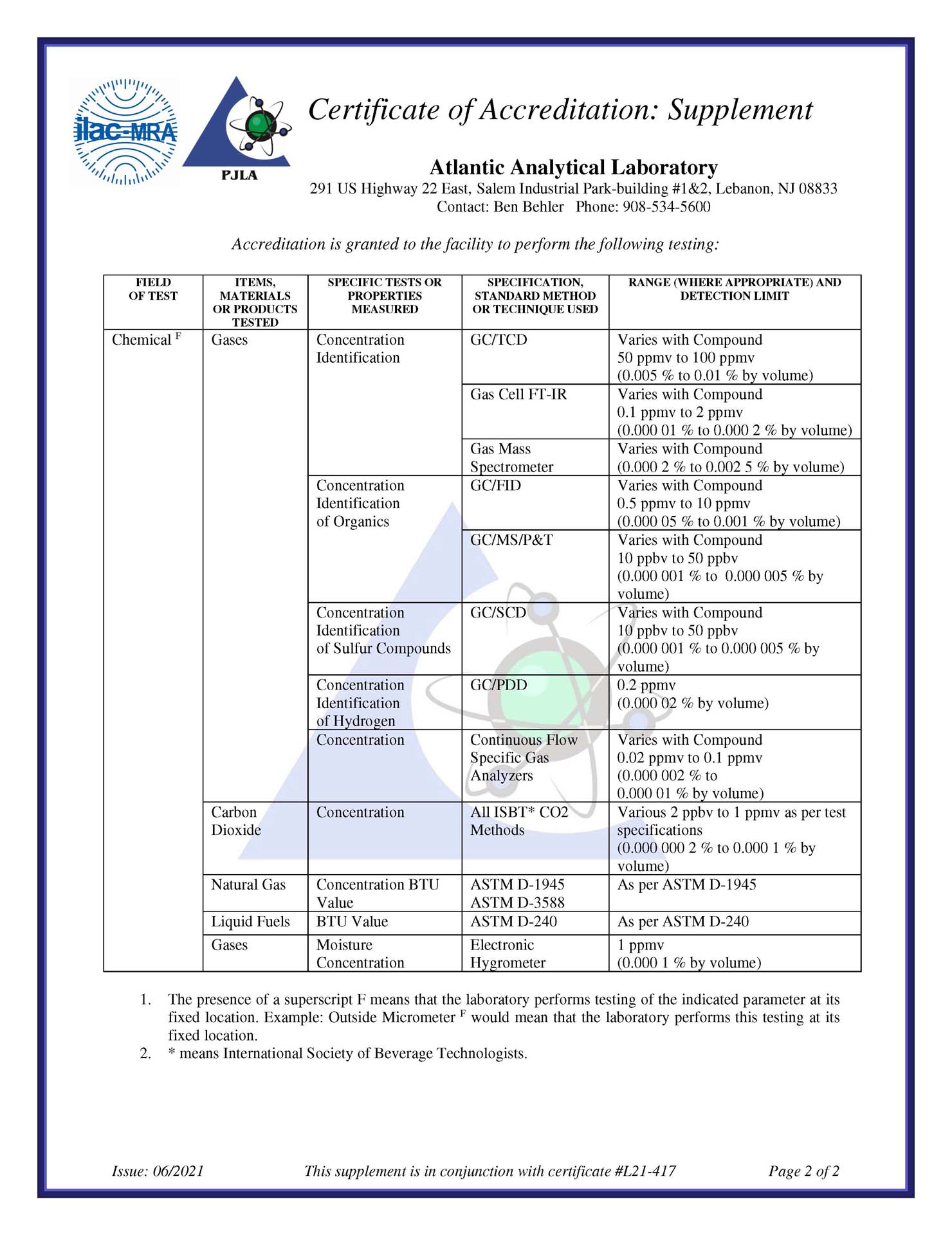
Compendial Gas Testing
Our facility dedicated to “current Good Manufacturing Practice” (cGMP) attests to our rapidly expanding pharmaceutical services and capabilities. Our facility specializes in developing and validating analytical methods designed for identifying pharmaceutical gases, volatiles and their impurities.
AAL offers many compendial gas testing methods that cover both the “United States Pharmacopeia/National Formulary” (USP/NF) and “European Pharmacopeia” (EP) monographs, plus the technical capability to validate custom specifications of gases and gas mixtures according to cGMP criteria.
By working together today, Atlantic Analytical can help pharmaceutical and biotech manufacturers anticipate and overcome the challenges of compliance with national and international regulatory processes of tomorrow in a cost-effective manner. Utilizing AAL’s out-source services as your contract research firm can add significantly to the overall project profitability. New regulatory challenges can blindside the pioneer, but for those who plan wisely and meet those hurdles today, compliance can offer greater rewards.
Visit our contact page or give us a call today to get started!