Well-Designed, Reliable, Analysis Strategies
Purity Testing
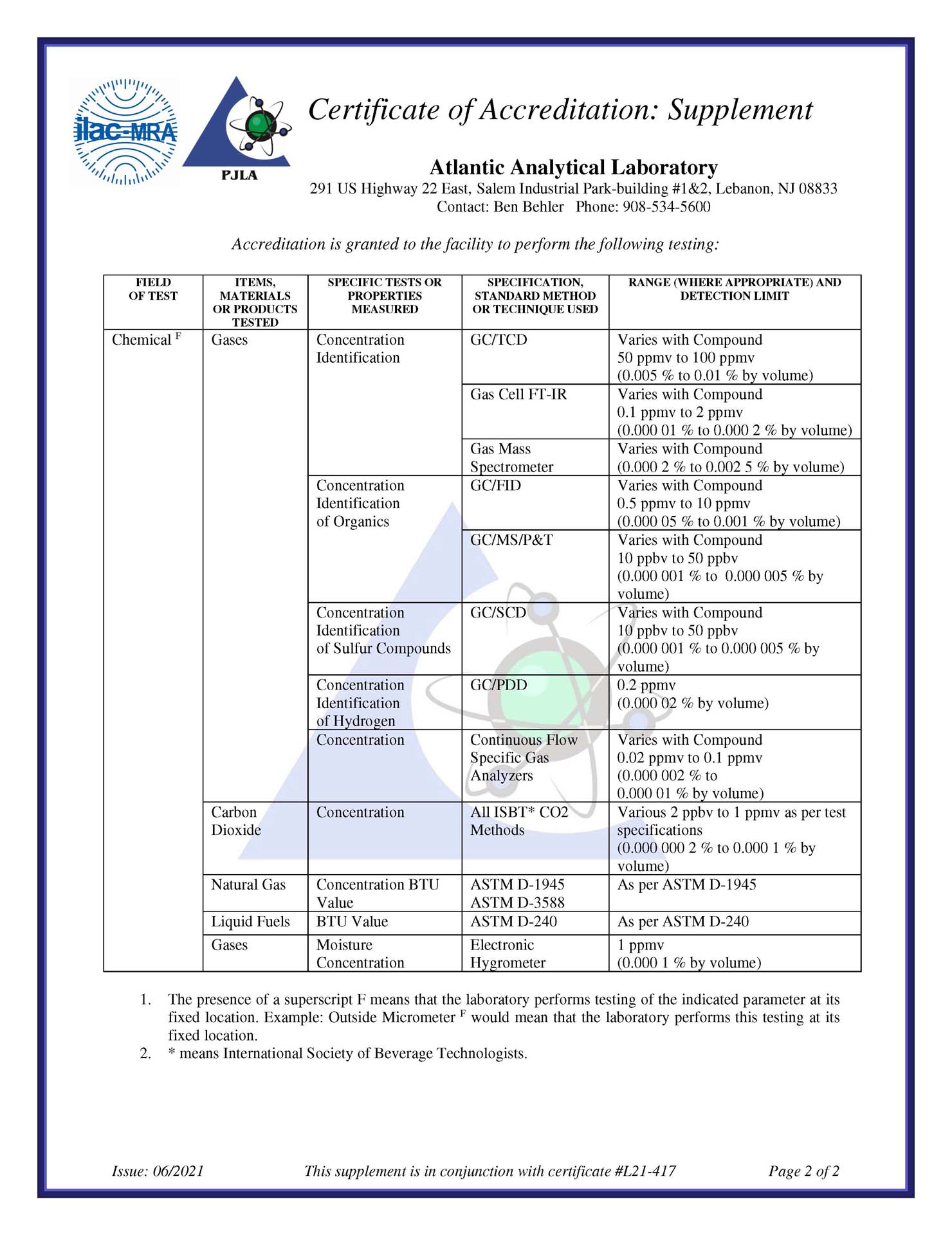
Many commercial gases; such as Nitrogen, Oxygen, Carbon Dioxide, Medical Air, Argon, Nitrous Oxide and specialty gas mixtures, are available in a range of purity grades. These gases may be used in the pharmaceutical industry as excipients, propellants or drug substances. The proper grade for a given application depends on many factors, and is often established by domestic and international regulatory agencies, namely the USP/NF and EP. The fully compliant analytical program that Atlantic Analytical offers to each client is designed to detect and quantify the critical contaminants associated with their selected grade of gas. A well-designed, reliable, analysis strategy ensures that undesirable contaminants have been monitored; and that the key gas components in a specialty mixture are present at their required concentrations.
Visit our contact page or give us a call today to get started!
Beyond Chromatographic And Spectroscopic Methods
Impurities Testing
Even at trace levels, unwanted chemicals in pharmaceutical ingredients may greatly influence a drug product’s efficacy and safety. While researchers strive to eliminate or control impurities, they rely on fast analytical tools with high sensitivity and specificity to better detect, identify, quantify, and characterize them. The technology for impurity analysis has improved beyond the traditional chromatographic and spectroscopic methods. Scientists are gaining an ever-clearer picture of their materials’ constituents. In some cases, technology is revealing never-before-seen impurities even in compounds thought to be well understood.
Better information about pharmaceutical impurities is generating new questions. What is the best way to handle them? How should we report them? How do they affect overall product quality? How should control procedures be put in place? Ingredient suppliers are working and debating with regulatory agencies to develop guidelines designed to eliminate redundant testing for APIs, explain the composition of excipients, and reasonably control the impurities in both.